Thumbnail

Dummy Blog 1
Release 17th February 2026
The Urgency of Propellant Transition
The 2025 HFC phasedown regulations have created an immediate challenge for pharmaceutical formulators developing pressurised metered dose inhalers (pMDIs). As traditional HFC-134a faces restricted availability, development teams must evaluate low-global warming potential (GWP) alternatives—but the two leading candidates, HFO-1234ze and HFA-152a, present fundamentally different formulation profiles. This isn’t a simple substitution exercise. Each propellant demands distinct approaches to API solubility, excipient compatibility, and device integration. With regulatory deadlines approaching and reformulation timelines extending 18-24 months, the propellant selection you make today will determine whether your product reaches patients on schedule. This guide compares the practical formulation considerations that matter most to development teams navigating this transition.
What Is HFO-1234ze?
HFO-1234ze (trans-1,3,3,3-tetrafluoropropene) represents the hydrofluoroolefin class of propellants specifically developed as an environmentally sustainable alternative to traditional HFCs. With a GWP of less than 1—comparable to carbon dioxide—HFO-1234ze addresses climate concerns whilst maintaining pharmaceutical-grade purity standards required for inhalation products.
Physical and Chemical Properties
HFO-1234ze exhibits a vapour pressure of approximately 4.7 bar at 20°C, notably lower than HFC-134a’s 5.7 bar. This difference affects valve design, actuator force requirements, and ultimately the aerosol plume characteristics your patients will experience. The propellant’s density (1.16 g/mL at 25°C) influences suspension formulation behaviour and particle settling rates during storage.
From a formulation perspective, HFO-1234ze’s polarity differs significantly from HFC-134a, which directly impacts API solubility. According to studies published in the International Journal of Pharmaceutics, HFO-1234ze generally demonstrates lower solvating capacity for many common respiratory APIs compared to HFC-134a, though this varies considerably by molecular structure (Stein et al., 2020). For suspension formulations, this reduced solubility can actually prove advantageous by minimising valve deposition and maintaining dose consistency.
Regulatory Status and Safety Profile
HFO-1234ze carries an A2L flammability classification under ASHRAE Standard 34, indicating low toxicity and low flammability under standard conditions. This classification requires specific handling protocols during manufacturing but remains acceptable for pharmaceutical applications. Both the FDA and EMA have provided pathways for HFO-1234ze-based pMDI approvals, with several products already granted marketing authorisation.
The propellant’s environmental credentials extend beyond GWP. Its atmospheric lifetime of approximately 16 days means minimal long-term accumulation, addressing both climate and ozone depletion concerns that drove the original CFC phaseout.
Formulation Advantages
- Reduced valve deposition due to lower API solubility in many cases
- Improved dose consistency over product lifetime in suspension formulations
- Compatibility with existing valve technologies with appropriate material selection
- Lower GWP than any other pharmaceutical propellant currently available
- Established regulatory pathway with precedent approvals in major markets
What Is HFA-152a?
HFA-152a (1,1-difluoroethane) has been investigated as a pharmaceutical propellant for over two decades, though commercial adoption has remained limited compared to HFC-134a. With a GWP of 138—significantly lower than HFC-134a’s 1,430 but higher than HFO-1234ze—it represents a pragmatic environmental improvement whilst offering distinct formulation characteristics.
Physical and Chemical Properties
HFA-152a demonstrates a vapour pressure of approximately 5.0 bar at 20°C, positioning it between HFO-1234ze and HFC-134a. This intermediate vapour pressure can facilitate device transition strategies, potentially allowing reformulation with minimal valve and actuator modifications. The propellant’s density (0.90 g/mL at 25°C) differs notably from both HFC-134a and HFO-1234ze, affecting suspension stability calculations and formulation density matching requirements.
The solvating properties of HFA-152a more closely resemble HFC-134a than those of HFO-1234ze, which can simplify reformulation programmes for APIs with established solubility profiles. Research in Respiratory Drug Delivery conferences has documented that many small molecule bronchodilators exhibit comparable solubility in HFA-152a and HFC-134a, though this must be verified for each specific API and excipient combination (Smyth et al., 2019).
Regulatory Status and Safety Profile
HFA-152a carries an A2 flammability classification, indicating higher flammability potential than HFO-1234ze’s A2L rating. This classification has historically created regulatory hesitation and requires enhanced safety protocols throughout manufacturing, storage, and distribution. Whilst technically feasible for pharmaceutical use, the flammability profile demands rigorous risk assessment and mitigation strategies.
The propellant has received regulatory approval for specific applications in certain markets, though adoption has been constrained by both flammability concerns and the subsequent development of lower-GWP alternatives.
Formulation Advantages
- Similar solvating properties to HFC-134a for many APIs
- Potential for simplified reformulation from existing HFC-134a products
- Intermediate vapour pressure facilitating device design continuity
- Lower cost compared to HFO-1234ze in current market conditions
- Established analytical methods adapted from HFC-134a programmes
Head-to-Head Comparison
| Parameter | HFO-1234ze | HFA-152a | Formulation Impact |
|---|---|---|---|
| Global Warming Potential | <1 | 138 | Regulatory preference increasingly favours HFO-1234ze; HFA-152a acceptable but less future-proof |
| Vapour Pressure (20°C) | 4.7 bar | 5.0 bar | HFA-152a’s higher pressure may simplify device transfer; both require valve optimisation |
| Density (25°C) | 1.16 g/mL | 0.90 g/mL | Significant difference affects suspension formulation and density matching strategies |
| Flammability Classification | A2L (low flammability) | A2 (flammable) | HFA-152a requires enhanced safety protocols; impacts manufacturing complexity and cost |
| API Solubility Profile | Generally lower than HFC-134a | Similar to HFC-134a | HFO-1234ze may require formulation optimisation; HFA-152a potentially simplifies reformulation |
| Regulatory Pathway | Well-established in EU/US | Case-by-case evaluation | HFO-1234ze has clearer regulatory precedent; HFA-152a faces additional scrutiny |
| Material Compatibility | Requires validation | Similar to HFC-134a | Both require compatibility testing; HFA-152a may leverage existing data |
These technical differences fundamentally shape development strategy, timeline, and risk profile. Our experience across multiple low-GWP formulation programmes demonstrates that propellant selection should occur during feasibility assessment, not after API synthesis or device selection. The downstream implications of choosing the wrong propellant can add 12-18 months to development timelines.
For teams evaluating these options, i2c’s formulation development services provide early-stage compatibility screening that identifies potential formulation challenges before significant resources are committed.
Formulation Challenges in Practice
Challenges with HFO-1234ze
The primary formulation obstacle with HFO-1234ze centres on API solubility optimisation. Whilst reduced solubility benefits suspension stability, it can complicate solution formulation development for APIs requiring dissolved delivery. Our formulation work has identified several practical strategies:
- Co-solvent screening to enhance API solubility whilst maintaining pharmaceutical acceptability
- Excipient selection optimised for HFO-1234ze’s polarity profile
- Particle engineering to achieve optimal aerodynamic properties in suspension formulations
- Valve material compatibility testing to prevent extractables/leachables issues
The lower vapour pressure requires attention to spray pattern characteristics and plume geometry. In our experience, formulation teams often need to iterate valve orifice dimensions and actuator design to achieve equivalent dosing performance to HFC-134a products.
Analytical testing becomes particularly critical with HFO-1234ze formulations. Standard methods developed for HFC-134a may require modification to account for different evaporation rates and density effects on cascade impaction results.
Challenges with HFA-152a
The flammability profile represents HFA-152a’s most significant practical challenge. Manufacturing facilities require enhanced safety systems, including explosion-proof equipment, appropriate ventilation, and rigorous process hazard analysis. Material compatibility testing must account for HFA-152a’s specific chemical properties. Comprehensive compatibility programmes should evaluate:
- Elastomer swelling and mechanical property changes
- Valve component extractables under accelerated conditions
- Container material interactions during long-term storage
- Actuator material compatibility across temperature ranges
For programmes prioritising speed to clinic, our Fast2Clinic service enables rapid formulation iteration whilst maintaining appropriate safety protocols for either propellant option.
Decision Framework: Choosing Your Propellant
Choose HFO-1234ze When:
- Environmental sustainability is a priority – The near-zero GWP provides maximum future security
- Your API demonstrates adequate solubility – Or when suspension formulation is appropriate
- You’re developing a novel device platform – Opportunity to optimise valve and actuator design
- Long-term market presence is planned – Best positions products for evolving regulations
Choose HFA-152a When:
- Reformulation timeline is critical – API solubility similarities to HFC-134a may accelerate development
- Your facility has existing flammability management – Capital investment is already in place
- Device transfer is a priority – The intermediate vapour pressure facilitates minimal valve modifications
- Cost sensitivity is significant – Current market pricing favours HFA-152a
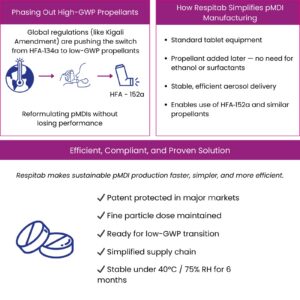
i2c’s patented Respitab® technology specifically addresses formulation challenges with both HFO-1234ze and HFA-152a, providing a proven platform for sustainable pMDI development.
Conclusion
The transition from HFC-134a to low-GWP propellants represents more than regulatory compliance—it’s an opportunity to optimise formulation performance whilst addressing environmental concerns. HFO-1234ze and HFA-152a each offer distinct advantages, but neither provides a universal solution. Your optimal choice depends on API properties, device requirements, regulatory strategy, and development timeline constraints.
At i2c, we’ve supported pharmaceutical companies through successful low-GWP propellant transitions for over three decades. Our integrated capabilities—from formulation development through gamma scintigraphy validation—enable comprehensive development programmes under one roof.
Ready to evaluate HFO-1234ze or HFA-152a for your specific formulation? Contact our formulation team for a technical consultation on your API and device requirements, or download our comprehensive whitepaper on low-GWP propellant formulation strategies.
Frequently Asked Questions
Can I simply replace HFC-134a with HFO-1234ze or HFA-152a without reformulation?
No. Whilst some formulation components may transfer directly, the different physical properties—particularly vapour pressure, density, and solvating characteristics—typically require formulation optimisation and device adjustment.
Which propellant will regulators prefer?
Regulatory agencies don’t mandate specific propellant selection, but HFO-1234ze’s superior environmental profile (GWP <1) increasingly influences regulatory and reimbursement discussions.
How long does propellant transition typically require?
Development timelines vary considerably, but expect 18-24 months from propellant selection through clinical batch manufacture for a well-planned programme.
Are there other low-GWP propellant options beyond these two?
HFO-1234ze and HFA-152a represent the most developed pharmaceutical propellant alternatives currently. For programmes requiring near-term market entry, these two options dominate practical consideration.
What compatibility testing is essential before committing to a propellant?
At minimum, conduct API solubility screening, preliminary stability assessment, valve component compatibility testing, and spray pattern characterisation.
Suggested Alt Text for Images:
- “Molecular structure comparison of HFO-1234ze and HFA-152a propellants showing chemical differences”
- “Side-by-side comparison chart of vapour pressure, GWP, and density for low-GWP pMDI propellants”
- “pMDI formulation development workflow showing propellant selection decision points”
- “Graph showing API solubility profiles in HFO-1234ze versus HFA-152a versus HFC-134a”
- “i2c Respitab technology diagram illustrating sustainable propellant formulation approach”
Related Content Recommendations for Content Cluster:
- “Navigating HFC Phasedown Regulations: A Timeline for pMDI Developers”
- “How Respitab® Technology Enables Sustainable Inhaler Development”
- “Propellant Compatibility Testing Protocols: What Formulation Teams Need to Know”
- “From HFC-134a to Low-GWP Alternatives: Case Studies in Successful Reformulation”
- “Analytical Method Development for HFO-1234ze Formulations”
